Friday, May 3, 2013

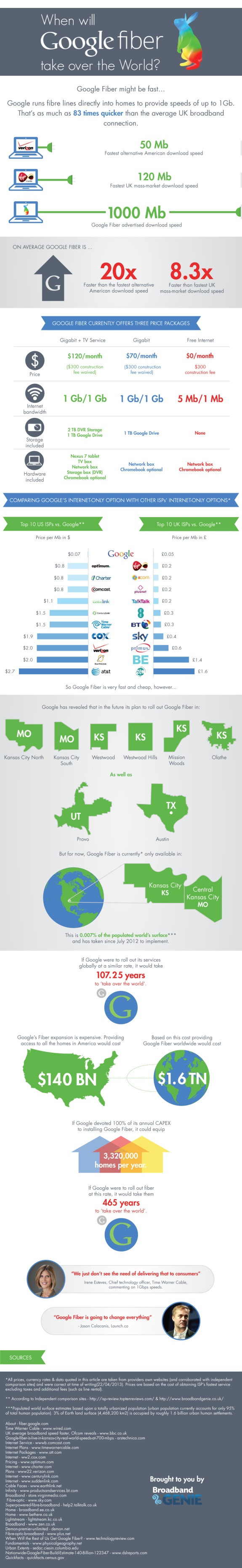
This is reassuring: the FDA says that, sometime this year, they'll finally get around to properly investigating triclosan, the germ-killing ingredient used in anti-bacterial soaps, mouthwash, toothpaste and toys. But how long has this potentially-harmful chemical been on the market? Only 40 plus years.
The FDA's investigation, which it hopes to complete this year, was spurred by pressure from lawmakers and consumer advocates after several animal tests showed various negative effects of triclosan. One 2009 study showed the chemical lowered testosterone and sperm levels in male rats and triggered early puberty in female rats, and a 2012 study found the chemical might restrict muscle contractions in fish and mice, which in humans could lead to weak heart and skeletal muscles.
"To me it looks like the risks outweigh any benefit associated with these products right now," said Allison Aiello, professor at the University of Michigan's School of Public Health.
Of course, the American Cleaning Institute denies that the chemical causes any harm.
"Triclosan is one of the most reviewed and researched ingredients used in consumer and health care products," says Brian Sansoni, a spokesman for the group, whose members include Colgate-Palmolive and Henkel Consumer Goods Inc., maker of Dial soap.
Among those who think Sansoni and his group are full of shit are 37 Kasier Permanente hospitals, who removed products containing triclosan in 2010, and Johnson and Johnson, who have pledged to remove the chemical from all its products by 2015.
The FDA first published drafts noting the chemical was "not generally recognized as safe and effective” in 1978. But they never finalized their results, so companies that had been using the chemical since 1973 were never forced to remove it from their products.
As it stands now, the FDA's website states that "the agency does not have evidence that triclosan in antibacterial soaps and body washes provides any benefit over washing with regular soap and water."
The Joy of Jury Duty
Why Americans should stop complaining and learn to appreciate this constitutional obligation

Americans who did not show up for jury duty as mandated raise their hands in response to a question from Circuit Judge Gregory Holder, at a lecture on civic responsibility in Tampa, Florida on November 4, 2011. (Skip O' Rourke/AP)
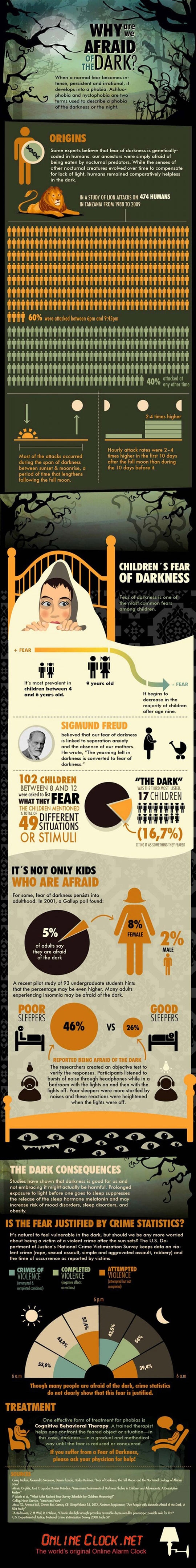
How can one appreciate an obligation? This is a question approximately 30 million Americans don't ask every year when they receive their jury summons because they are too busy grumbling about this core constitutional responsibility of citizenship. This is also a question of growing importance as courts are struggling to find enough jurors for trials.It all begins with a letter in the mail. "Dear Citizen," it reads. You hold in your hand an invitation. Sure, it uses the word "summons," and is probably not the kind of invitation you look forward to receiving. Yet, it is still an invitation -- an invitation to participate in the American experiment of self-government. And you can feel flattered that you have been invited. It means that you have not committed a felony (that anyone knows about), that you are mature enough to judge others, and that your community needs you. It's only polite to accept. And, it's even better to think about how you might enjoy the experience.
Turning the dread of jury duty into a form of enjoyment begins with understanding why jury duty matters. Simply put, it may well be the closest you ever come to the Constitution -- not just exercising a right it gives you, but participating in the process through which constitutional rights and values comealive in practice. In a country formed from a single founding document, it is amazing how disconnected most of us are from its meaning and purpose. Jury duty changes that reality - it is a day of constitutional connection. It is also a government-provided free pass from your normal family and work responsibilities. It is the law of the land that you cannot complete your workaday routine. Jury duty thus provides an opportunity (with plenty of waiting time) to reflect on our shared constitutional values.
What are these constitutional values? Participation, deliberation, fairness, equality, accountability, liberty, and the common good - these are constitutional values, and they are embedded in jury service.
A jury summons is an invitation to participation. Jurors are asked to involve themselves in some of the most personal, sensational, and terrifying events in a community. It is real life, usually real tragedy, played out in court. Jurors confront disturbing facts, bloody images, or heart-wrenching testimony. A jury may have to decide whether a man lives or dies, or whether a multimillion-dollar company goes bankrupt. A jury will have to pass judgment in a way that will have real-world effects on both parties before the court. This active role was not accidental. Participation in jury service teaches the skills required for democratic self-government. Being a juror lets you develop the habits and skills of citizenship.
What are these "democracy" skills? Think about what is required for a politically active nation. As a juror, you are asked to "vote" based on contested facts. You must debate issues framed by contesting parties. This involves listening to others and tolerating dissenting views (as well as expressing your own opinions). Jurors necessarily expand their social interaction with different types of people, broadening perspectives, contacts, and sources of information. To apply the law jurors must understand the law, the rights of the parties, and the legal rules guiding the decision. Each of these participatory skills--deliberation, debate, tolerance, cooperation, civility, legal decision making--is what we need for a democracy to work. The participatory aspect of jury duty shapes our constitutional character. Those habits and skills, our civic education, helps define who we are as Americans.
Or, as another example, take the value of deliberation. In the very first sentence of The Federalist Papers, a collection of essays and arguments in favor of the U.S. Constitution, Alexander Hamilton invited Americans to this different way of deciding, "You are called upon to deliberate on a new Constitution," he wrote (emphasis added). It was a call that perfectly fits the thinking of a democracy. Deliberation involves collective decision making--a willingness to think together using reason and informed discussion to come to a final decision.
Why is deliberation important? Because the process of deliberating--of sitting down and hashing out a problem with others--creates better thinkers and better decisions. As thinkers you become invested, informed, and connected. Such dynamic thinking forces you to consider different ideas and reason your way to a final decision. Through the process of deliberation, jurors are made aware of different viewpoints, sometimes even new worlds, as they are asked to judge life choices, industries, and realities that they may never have encountered before. Through jury instructions, jurors necessarily inform themselves about the legal system and the legal rules at play. Throughout the trial process, jurors develop the social mores necessary for success in other group activities. After all, if you can work with twelve people to agree on a verdict, you might be able to work together in a democracy.
Or as a final example, take the principle of equality. Throughout your jury service, you are known by a number--a juror number. You respond to that number. There are no nicknames or familiarities on jury duty. In the same way there are no titles. Whether you are a soccer mom or a Senator (or both), you are simply a number to the jury system. The number is not meant to insult, but to equalize. It provides the anonymity of being a citizen, one of millions who are doing exactly what you are doing in court: waiting for his or her number to be called.
Jury service allows you to see equality in action. In a world that is anything but equal, we tend to forget what equality feels like. You know your presidential vote counts as much as anyone else's, but you also know that the lobbyists, interest groups, and activists have more influence in the political process than your single vote. But in the jury room those differences become irrelevant. Whether you are a rocket scientist or rock guitarist, a linguist or laborer, jurors are given the same facts. Jurors see the same witnesses, hear the same arguments, and get an equal voice in the decision. Thus, the principle of one person, one vote, is actually observable on jury duty. This leveling mechanism strips away the divisions of our normal, unequal society. For a brief moment you see how democracy is supposed to work.
The invitation to jury service is thus an invitation to understand our most basic national principles. The simple fact is that jury duty is one of the few constitutional rights that every citizen has the opportunity to experience. It remains an American bond. It connects people across class, national origin, religion, and race. Jury experience exists as one of the remaining connecting threads in a wonderfully diverse United States. It links us to our founding principles and challenges us to live up to them. Every time you serve as a juror, you become closer to this constitutional spirit; and every time you reflect on and appreciate these principles, you strengthen our constitutional character. That is the joy of jury duty
.
Thursday, May 2, 2013
suitcase-stickers






SUITCASE STICKERS
These suitcase stickers are not for the faint of heart. In fact, The Cheeky even tells you that with this warning on the site: “Caution: Some of these stickers may cause offense to airport and immigration staff. But you would have figured that out whilst enjoying those cavity searches.” Putting a sticker on your suitcase that makes it looks like you’re toting a few keys of “sugar,” a stewardess, a pleasure chest or a trunk full of money could certainly (hell–probably) offend someone at the TSA. We’d like to think that they’d probably think it was funny and let you go, but if you chicken out before the security checkpoint and peel it off you’re only going to be throwing about ten bucks away. You could also put it on your buddy’s bag when he’s not paying attention. Not that we’d ever do that or anything.
Wednesday, May 1, 2013
A Dead-Simple Tip Jar That Takes Credit Cards
BARISTAS REJOICE! JUST BECAUSE CUSTOMERS ARE USING PLASTIC DOESN’T MEAN YOUR TIPS HAVE TO SUFFER.
It’s so easy to drop the loose change from your daily coffee purchase as a "thank you" for your favorite barista, but paying with plastic completely changes the transaction--oftentimes you don’t even have to sign, instead just cruising straight out the door with your morning cup. As cash purchases continue to wane, cafe aficionado (and empathetic soul) Ryder Kessler noticed that the servers at his local haunt weren’t getting tipped as much by credit-paying customers who would, normally, leave a little something behind. So he teamed up with his brother Judd to createDipJar--every counter employee’s new best friend.
The concept is simple; a single dip equals a single dollar. It’s separate from the regular payment, which eliminates the need for mental arithmetic on how much to give on a receipt, and it’s a quick in-and-out while the card’s still in your hand. Uniting a pared-down aesthetic with a no-brainer action was key to making the product a success; they capitalized on Judd’s economics PhD and various academic and behavioral research to refine the concept down to its essentials. “People are more likely to give when it’s made as easy as possible, and when a clear norm is articulated,” Ryder tells Co.Design. “So the biggest design decision was to eliminate any buttons or screens, and to pre-set the amount.” Once a retailer registers a DipJar, gratuities are distributed directly to the earning staff members.

Career Advice: Give
Givers focus on others, takers on themselves, and matchers care most about fairness. Studies show that most professional success, not just satisfaction, goes to givers.

Akintunde Akinleye/Reuters
In 1990, Jon Huntsman, Sr. made a business decision that most in corporate America would probably have called insane. He was intensely negotiating the biggest business deal of his life with Charles Miller Smith, the head of a British chemical company. Deep into the negotiations over the acquisition, Smith's wife died. She had been suffering from terminal cancer. It was unfortunate, but business is business and the negotiation was incomplete. On top of that, Huntsman had millions of dollars on the line -- money that would be his if he just pushed Smith further.But he didn't.
"I decided the fine points of the last 20 percent of the deal would stand as they were proposed," he later wrote. "I probably could have clawed another $200 million out of the deal, but it would have come at the expense of Charles' emotional state. The agreement as it stood was good enough."
When people are stressed out, their first instinct is to protect themselves -- or to retreat into a taker mentality. But operating like a giver may actually be more effective in buffering against stress and enhancing well-being.In his 2008 book Winners Never Cheat, Huntsman summarized his philosophy on business and life, writing, "Monetarily, the most satisfying moments in my life have not been the excitement of closing a great deal or the reaping of profits from it. They have been when I was able to help others in need ... There's no denying that I am a deal junkie, but I also have developed an addiction for giving. The more one gives, the better one feels; and the better one feels about it, the easier it becomes to give."
Huntsman is what organizational psychologist Adam Grant calls, in his provocative new book, a "giver." In Give and Take, Grant, the youngest tenured professor at the University of Pennsylvania's Wharton School of Business, wields a large body of social science research, much of it his own, to challenge the idea that career success is a zero-sum game in which your gains equal my losses, a harmful idea that discourages people from helping each other out at work.
In Grant's book, the reader encounters three types of people: givers, takers, and matchers. Matchers reciprocate favors and good deeds tit-for-tat. Takers try to tilt the balance of a transaction in their own favor, hoping to get more than they give -- the type of person who takes credit for someone else's work. Givers are the opposite. Their hallmark is generosity. Crudely put, givers are focused on others, takers are focused on themselves, and matchers care above all about fairness.
Most people are givers in their personal relationships. They act selflessly and try to contribute more than they take with those they love. But when these people enter the workforce, their style of interacting with others changes dramatically. As Grant told me in an interview, "There is an extraordinary number of people who are in a giver mindset at home and a matcher or taker mindset in the work setting." Only 8 percent of people describe themselves as givers at work. That's because most people think it's safer to operate like a taker or matcher at work; givers, they think, are chumps who will fall behind in the game of life.
Across four other studies, researchers found that giving time away -- in the form of volunteering -- makes people feel like they actually have more time than if they spent time on themselves, wasted time, or got a random bit of free time.Grant explodes that myth in his book, showing that givers are among the most successful people in business. They may also be the happiest. "There is powerful evidence," Grant tells me "that givers experience more meaning in their work than takers or matchers."
This is important considering that Americans spend most of their waking hours -- most of their lives -- at work. The average American man works 8.4 hours per day and the average American woman works 7.7 hours a day. How they feel in those hours is a major determinant of their well-being. But, according to the American Psychological Association, nearly 70 percent of Americans cite work as a major source of stress in their lives and four out of ten say that they experience stress at work on a daily basis. Onereport indicates that over half of working Americans are unsatisfied and unhappy with their jobs. The top person people don't like being around is, according to the National Time Use survey, their boss. Bosses and work seem to be significant sources of unhappiness for many people.
When people are stressed out, their first instinct is to protect themselves -- or to retreat into a taker mentality. But operating like a giver may actually be more effective in buffering against stress and enhancing well-being. On its face, this is counter-intuitive. Time is a scarce resource, especially for people who are stressed. Being a giver involves taking time away from yourself to help someone else. This could seemingly aggravate stress levels, but it actually alleviates them.
In one study, Grant and a colleague found that givers who were high school teachers were less vulnerable to stress and exhaustion if they saw the impact their giving was having on their students. Across four other studies, researchers found that giving time away -- in the form of volunteering -- makes people feel like they actually have more time than if they spent time on themselves, wasted time, or got a random bit of free time.
Not only does being a giver protect against stress, but it also has lasting benefits on well-being outside of work. In a study of 68 firefighters, Grant and a colleague found that those who helped others on the job felt happier at home at bedtime than those who did not. Interestingly, the increase in happiness from giving was delayed. The firefighters were not any happier at the end of the working day, but only after they had been at home for several hours.
Another study, led by psychologist Sonja Lyubomirsky, shows that the more giving an individual is, the happier he becomes. Participants were randomly assigned to one of two conditions: Over a six-week period, they would either perform five random acts of kindness all in one day or to do one act of kindness across five different days. Those who fit all five acts of giving into one day were happier at the end of the study than those who thinly spread their giving out.
Being a giver is a principle many of us apply -- or at least try to -- in our personal lives. It's also one we should internalize in the workplace. As Grant puts it, "Let's ignore the evidence that givers often outperform matchers and takers. Let's say their scores are even. Given that, here is the question I would ask takers: Would you rather achieve success in ways that come at the expense of others or in ways the lift other people up?" He also points out that if you try to be a giver just to get ahead, it probably won't work
.
Tuesday, April 30, 2013
An Ingenious Cookbook Uses Infographics Instead Of Words
EVER GET LOST IN A COOKBOOK’S LONG NARRATIVE OF INSTRUCTIONS? US TOO. ONE DESIGNER/ILLUSTRATOR BOILED 50 RECIPES DOWN TO SIMPLE SKETCHES.
How do you make lasagna? Even though it’s not that complex of a dish, to spell out the methodology--the specific ingredients and the many small, easy steps of prep work--it would take me half a page of type or more.
But for designer/illustrator Katie Shelly, writer of Picture Cook: See. Make. Eat., the recipe for lasagna looks a lot different. It’s a simple sketch that deconstructs lasagna into its discrete components. So with a glance, anyone can learn how to layer cheese, noodles, sauce and meat to make the dish.

Of course, illustration isn’t a new idea in cookbooks--drawings that show finder details of technique like dicing onions are mainstays in classic food tomes. Where Shelly’s illustrations become radical is their scope. Using a bare minimum of text, she depicts everything from a quickly blended Gazpacho to a 2-hour, 21-ingredient pho. The somewhat oddball idea came to Shelly when writing down a friend’s eggplant parmesan recipe over the phone.
“She started by saying ‘well first you get out three bowls …’ and so it was natural to just draw the three bowls in that moment, and then I stuck with drawing the rest of the recipe on this little scrap of paper,” Shelly tells Co.Design. “That night when I got down to cooking, I pulled out the drawing of the recipe and found that it was really useable, more useable than a text recipe where you have to stop what you’re doing and read and re-read the steps.”

From there, she pushed the idea further, drawing on a background in UX testing to create recipes and spot where her testers (friends) may may potentially go wrong. This honed her craft, so much so that you’ll probably be a little impressed by her subtle uses of iconography (like the number of flames below a pot slyly depicting relative temperature), but you’ll be downright shocked at her ability to make even the most involved from-scratch cooking look fun and approachable.
“One of my goals for this book is to encourage people to just let go of rigid kitchen rules and be loose and free about cooking. The charm of the illustrations is great for its own sake, but it’s also performing a real function in helping make cooking feel easy, lighthearted, and do-able,” Shelly writes. “So what if you mess up? Once things get too serious, the drawings stop being inviting and start seeming intimidating. Like how people feel so oppressed by Ikea diagrams.”
Picture Cook won’t be released until October. But you can preorder it today for all of $11 on Amazon.
Monday, April 29, 2013
Subscribe to:
Comments (Atom)